Making certain that biosafety testing and characterization assays are scientifically sound and meet cGMP rules is a fancy course of action that needs multifaceted experience, and often results in a big expense of your time and sources.
Annual gowning certification is additionally expected. In the course of this method, microbiologists ought to stick to strict aseptic gowning procedures, with RODAC™ agar plates accustomed to sample unique gown spots.
Organic products or biologics could be made up of sugars, proteins, or nucleic acids, or a mix of these substances; plus they may be residing entities, for instance cells and tissues. Organic products are made by biotechnology methods in industrial quantities. Biological products can even be known as biopharmaceuticals.
Irrespective of rigid adherence to guidelines and most effective techniques, sterility testing can current worries. Some popular troubles incorporate:
Sterility indicators are made use of to check the standard and monitoring of sterilization procedures. They are able to suggest whether or not microbial development occurs or sterilization was helpful. There are various different types of sterility indicators for various sterilization methods such as dry warmth, moist warmth, gaseous, radiation, and filtration sterilization.
Sampling is described given that the statistical course of action of choosing a part or percentage of a complete merchandise batch to signify your entire batch. And samples are picked or picked inside a random manner to serve as consultant samples of the whole lot. Using membrane filtration system and direct inoculation employing tradition (nutrient) media tend to be the two most significant principal sterility testing technique utilised to find out the sterility of an item.
Sterility testing could be carried out working with various methods and procedures, depending on the item style and regulatory necessities. The 2 Major methods Utilized in sterility testing are membrane filtration and immediate inoculation.
Clinical equipment Medical units that happen to be very likely to are available in immediate or indirect contact with sterile system places are required to undertake sterility testing.
Top quality Regulate really should be a basic section of parenteral products manufacturing. All of the 4 primary assessments which are executed are critical and have its have relevance in parenteral creation.
Sterility testing is usually a stringent procedure that will involve deciding the absence of viable microorganisms in pharmaceutical products. This testing plays a significant purpose in guaranteeing the sterility and protection of medications, injectables, and professional medical equipment.
Should your fast sterility testing technologies is novel, there’s nothing at all like a robust human body of peer-reviewed journals that show your technological know-how to assist persuade FDA reviewers that it’s scientifically seem.
Nevertheless, a adequate amount of products samples from Every batch of your products are subjected to sterility testing in order to give
With decades of expertise and probably the most detailed household of diagnostic options, bioMérieux understands the difficulties facing dairy manufacturers – and the restrictions of these days’s tests. That is definitely why we created AUGMENTED DIAGNOSTICS to detect, watch and look into at just about every action of the lower-humidity production method. This allows for laboratory procedures to become automatic and to operate at an best stage, although also being really Price-successful.
three. High check here quality Management: Implementation of sturdy top quality control actions, which include common calibration of apparatus, schedule testing of lifestyle media, and checking of incubation situations, is vital for guaranteeing the dependability and accuracy of sterility testing final results.
 Ben Savage Then & Now!
Ben Savage Then & Now! Richard "Little Hercules" Sandrak Then & Now!
Richard "Little Hercules" Sandrak Then & Now!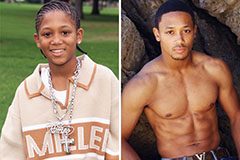 Romeo Miller Then & Now!
Romeo Miller Then & Now! David Faustino Then & Now!
David Faustino Then & Now!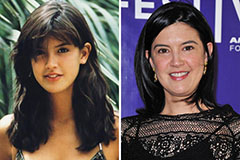 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!